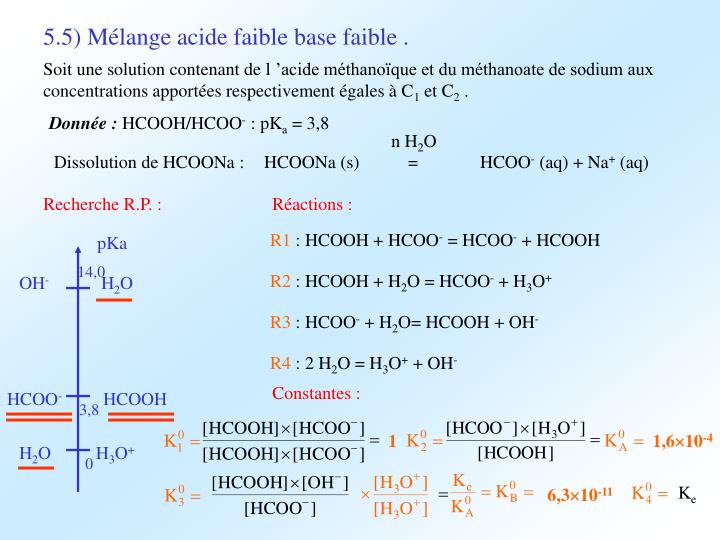
Find the equilibrium constant equilibrium HCOO + H2O + HCOOH + OH- In a solution of 0.1 M HCOONa. K (HCOOH) = 1.8 x 104 (1) 1.8 x 10-4 (275.56 10 (3) 5.56 x 10-11 (4) 1.8 x 10-18

SOLVED: Calculate the pH of a solution prepared by dissolving 0.270 mol of formic acid (HCOOH) and 0.230 mol of sodium formate (HCOONa) in water sufficient to yield 1.00 L of solution.

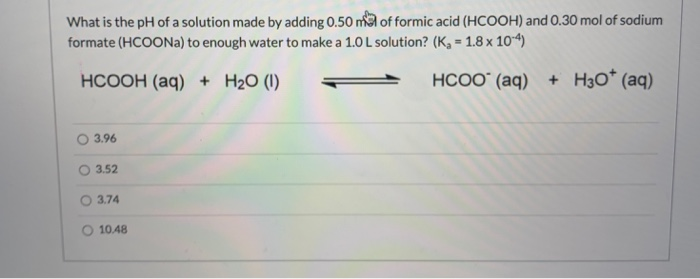
SOLVED: A 1.0 L buffer solution is prepared with 0.25 M formic acid (HCOOH) and 0.50 M sodium formate (HCOONa) - HCOOH(aq) + H2O(aq) = HCOO-(aq) + H3O+(aq). pKa = 3.74. What

The aqueous solutions of HCOONa, C_(6)H_(5)NH_(3)CI, and KCN are, respectively, | 12 | IONIC EQ... - YouTube

Find the equilibrium constant equilibrium HCOO + H2O HCOOH + OH- In a solution of 0.1 M HCOONa. Ka(HCOOH) = 1.8 * 104 (1) 1.8 x 10-4 (2) 5.56 x 10° (4) 1.8 * 10-18 (3) 5.56 x 107-11

Find the equilibrium constant equilibrium HCOO + H2O HCOOH + OH- In a solution of 0.1 M HCOONa. Ka(HCOOH) = 1.8 * 104 (1) 1.8 x 10-4 (2) 5.56 x 10° (4) 1.8 * 10-18 (3) 5.56 x 107-11
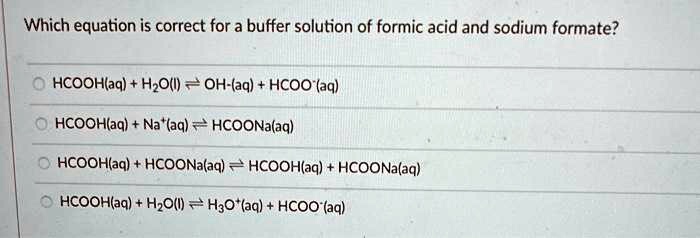
SOLVED: Which equation is correct for a buffer solution of formic acid and sodium formate? HCOOH(aq) + H2O(l) ⇌ H3O+(aq) + HCOO-(aq) HCOOH(aq) + NaOH(aq) ⇌ HCOONa(aq) + H2O(l) HCOOH(aq) + HCOONa(aq)

SOLVED: The neutralization of formic acid with aqueous NaOH produces sodium formate (HCOONa) as the only product. The reaction can be represented as follows: HCOOH + NaOH -> HCOONa + H2O.

Reductive Hydrodehalogenation of Halogenated Carboxylic Acid Derivatives Using a DMSO/HCOONa·2H2O System | Organic Letters