H2O2=H2O+O2 balance the chemical equation @mydocumentary838. h2o2=h2o+o2 balance the equation. - YouTube

Hydrogen peroxide (H2O2) can act as oxidising as well as reducing agent in both acidic and alkaline media. From the following given reactions, select the option(s) in which reducing action of H2O2

Role of H,O, in the following reactions is respectively (i) H202 +03 - H20 + 202 (ii) H202 + Ag,0 + 2Ag + H2O + O2 Options: X Oxidising in (i) and
![80] Decomposition of hydrogen peroxide is given by the following reaction; 2H2O2 + 2H2O + O2 The proposed mechanism is n ways Rate H2O2-H2O + O (slow) determining H2O2+0 → H2O + 80] Decomposition of hydrogen peroxide is given by the following reaction; 2H2O2 + 2H2O + O2 The proposed mechanism is n ways Rate H2O2-H2O + O (slow) determining H2O2+0 → H2O +](https://toppr-doubts-media.s3.amazonaws.com/images/2785739/3a8762db-6023-4a5c-9588-862513a5c19d.jpg)
80] Decomposition of hydrogen peroxide is given by the following reaction; 2H2O2 + 2H2O + O2 The proposed mechanism is n ways Rate H2O2-H2O + O (slow) determining H2O2+0 → H2O +
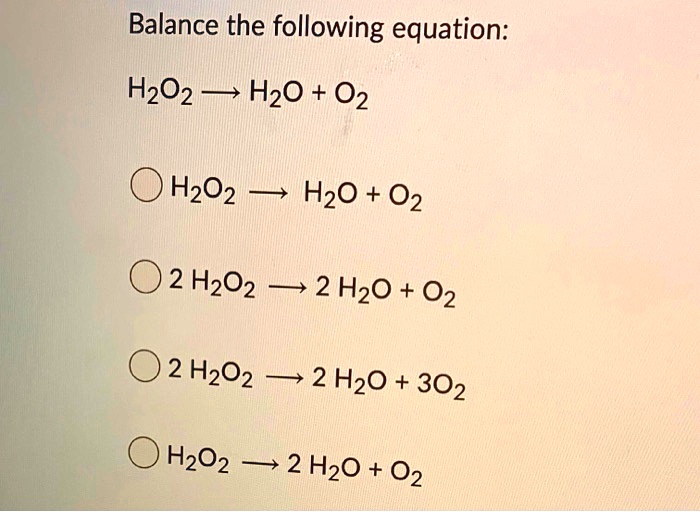
SOLVED: Balance the following equation: H2O2 H2O + O2 H2O2 H2O + O2 2 H2O2 2 H2O + O2 2 H2O2 2 H2O + 3 O2 H2O2 2 H2O + O2

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Comment équilibrer : H2O2 → O2 + H2O (peroxyde d'hydrogène, dioxygène, eau) | Physique-Chimie - YouTube

H2O2=H2O+O2 balance the chemical equation @mydocumentary838. h2o2=h2o+o2 balance the equation. - YouTube

2H202 alkaline medium *2H20 + 02 the proposed mechanism is as given below : (1) H2O2 +1 → H2O+IO (slow) (2) H202 + 10 + H20+1+02 (fast) (i) Write rate law the
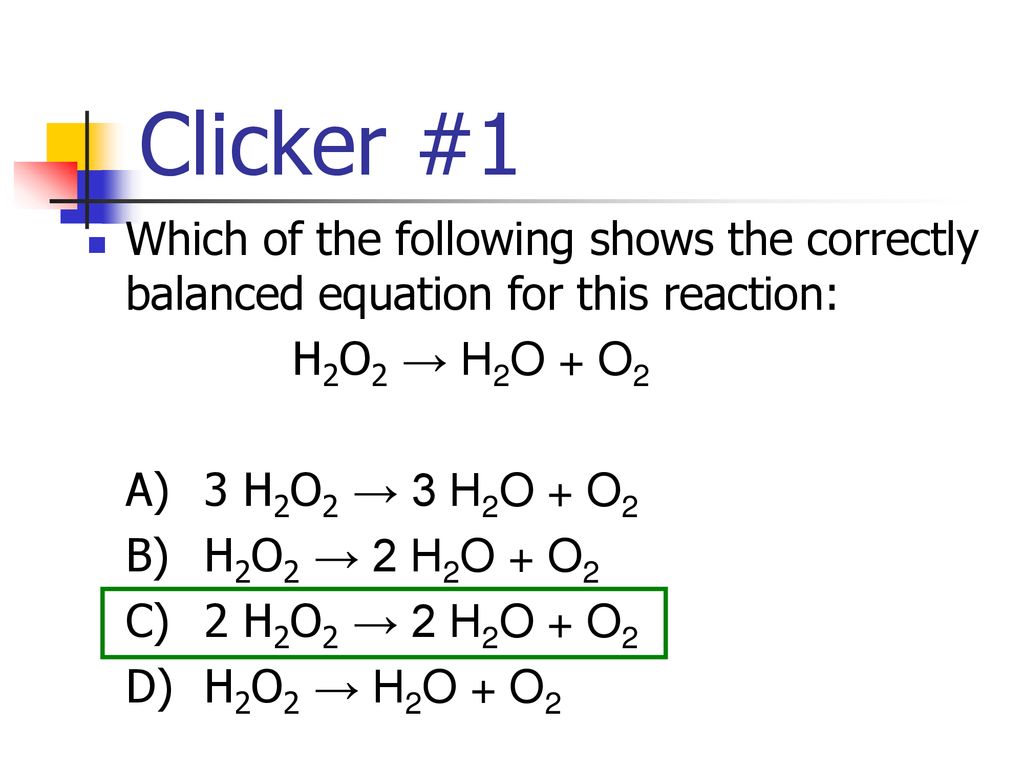
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download
1) H2O2 + O3 → H2O +2O2 2)H2O2 +Ag2O →2Ag +H2O +O2 Determine whether H2O2 is oxidised or reduced in the above reaction? Explain.
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as