
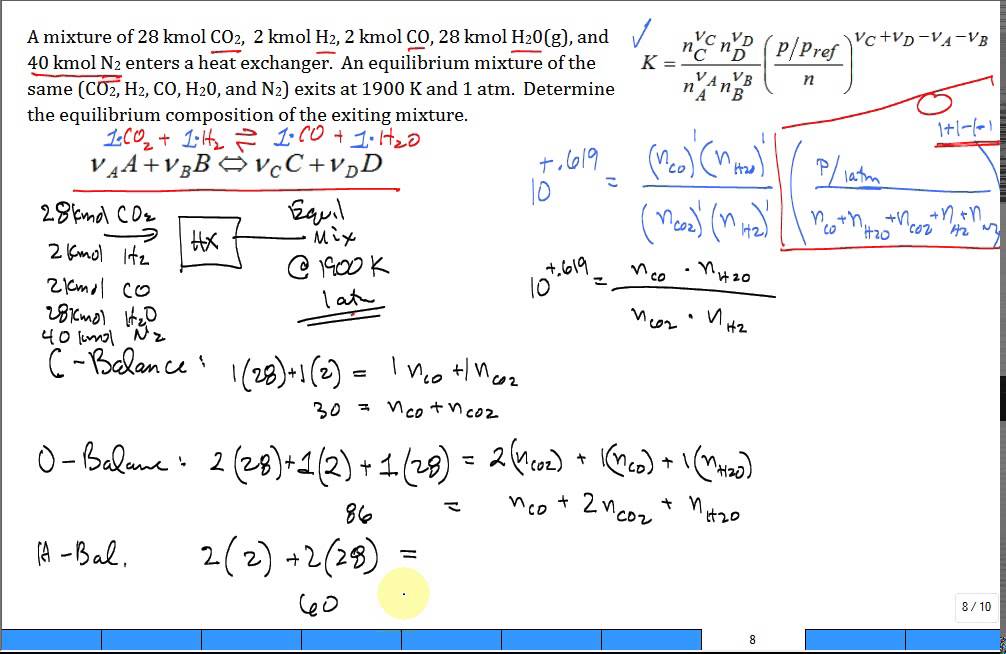
CO-ADSORPTION OF CO, H2O AND MECHANISM OF WATER GAS SHIFT REACTION ON ZnO CATALYST SURFACE: A DENSITY FUNCTIONAL THEORY STUDY | Semantic Scholar

SOLVED: What is the total energy change for the following reaction: CO + H2O -> CO2 + H2? Given: C-O bond: 358 kJ/mol H-O bond: 463 kJ/mol H-H bond: 436 kJ/mol

Reaction of CO, H2O, H2 and CO2 on the clean as well as O, OH and H precovered Fe(100) and Fe(111) surfaces - Catalysis Science & Technology (RSC Publishing)

For the reaction: Co(H2O)6^{2+} + 4Cl^- \leftrightharpoons CoCl4^{2-} + 6H2O After adding 12M HCl, what would be the roles of H^+ ions and Cl^- ions? | Homework.Study.com
CO2+H2=CO+H2O, 1 mole of CO2 and 2 moles of H2 are placed in a 2L container. At equilibrium, the concentration of CO is 0.28 mol/L. What is the equilibrium constant Kc for
Kp for the reaction CO2 + H2 =CO + H2O is found to be 16 at a given temperature. Originally equal number of moles of H2 and CO2 were placed in the
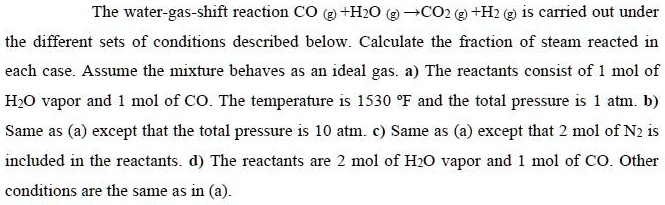
SOLVED: The water-gas-shift reaction CO (g) + H2O (g) â†' CO2 (g) + H2 (g) is carried out under different sets of conditions described below. Calculate the fraction of steam reacted in

64 The equilibrium constant the reaction, CO(g) + H2O (9) CO2 (g) + H2 (g) a certain temperature is 2.2. Initially one mole of CO and one mole of H2O are placed

I 6.4 The equilibrium constant the reaction, CO + H20 (g) CO2 (g) + H2 (9) a certain temperature is 2.2. Initially one mole of CO and one mole of H2O are

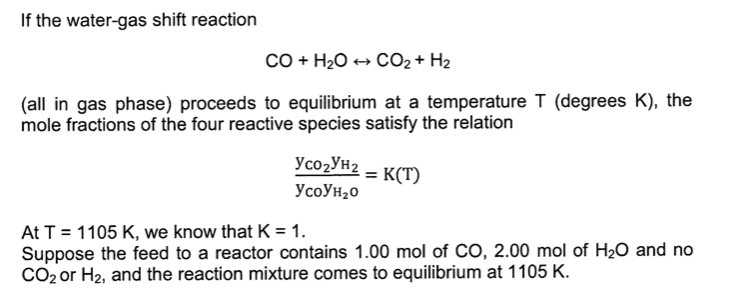
For the equilibrium CO + H2O CO2 + H2 .The relation between Kp and Kc at 25^o C and at 100^o C are: - Brainly.in

![Co(H2O)6]2+ Co(H2O)6]2+](https://tp-inorga-1-13.webself.net/file/si532904/CoH2O6-fi8185737x250.png)


![co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why](https://cdn.eduncle.com/library/scoop-files/2021/9/can_image_1631894248696.jpg)

![Co(H2O)6]2+ Co(H2O)6]2+](https://tp-inorga-1-13.webself.net/file/si532904/Spectre%20Co6-fi8187013x610.png)
![Invisible Ink 2[Co(H2O)6]Cl2(s) Co[CoCl4](s) + 12 H2O - ppt download Invisible Ink 2[Co(H2O)6]Cl2(s) Co[CoCl4](s) + 12 H2O - ppt download](https://slideplayer.com/16323544/95/images/slide_1.jpg)