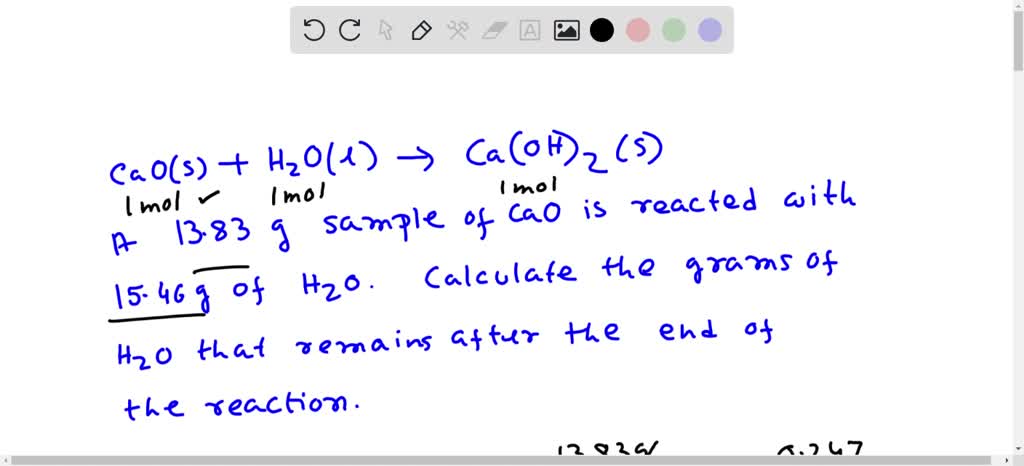
SOLVED: Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + H2O(l) → Ca(OH)2 (s) A 13.83 g sample of CaO is reacted with 15.46 g of
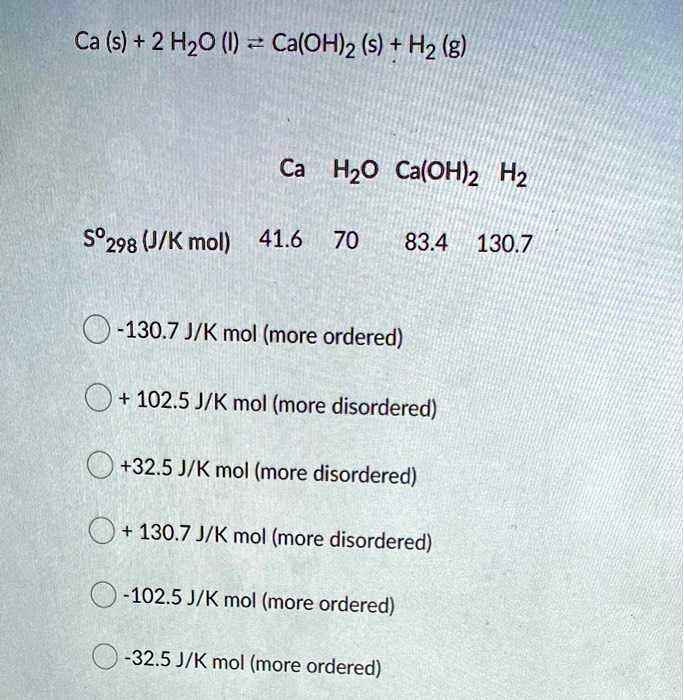
SOLVED: Ca (s) + 2 H2O (l) = Ca(OH)2 (s) + H2 (g) Ca H2O Ca(OH)2 H2 592.98 (J/K mol) 41.6 70 83.4 130.7 413.07 J/K mol (more ordered) 102.5 J/K mol (

Schematic diagram of CaO/Ca(OH)2 chemical heat pump: (a) heat storing... | Download Scientific Diagram

If 32.5 grams of CaO are dissolved in 212 grams of water, what is the concentration of the solution in percent by mass? | Socratic

How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) | How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) Hi Guys, welcome back to








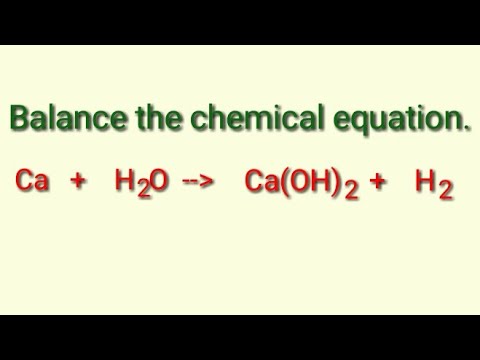




![Punjabi] Balance the following equation : Ca + H2O → Ca(OH)2 + H2 Punjabi] Balance the following equation : Ca + H2O → Ca(OH)2 + H2](https://static.doubtnut.com/ss/web-overlay-thumb/10303062.webp)