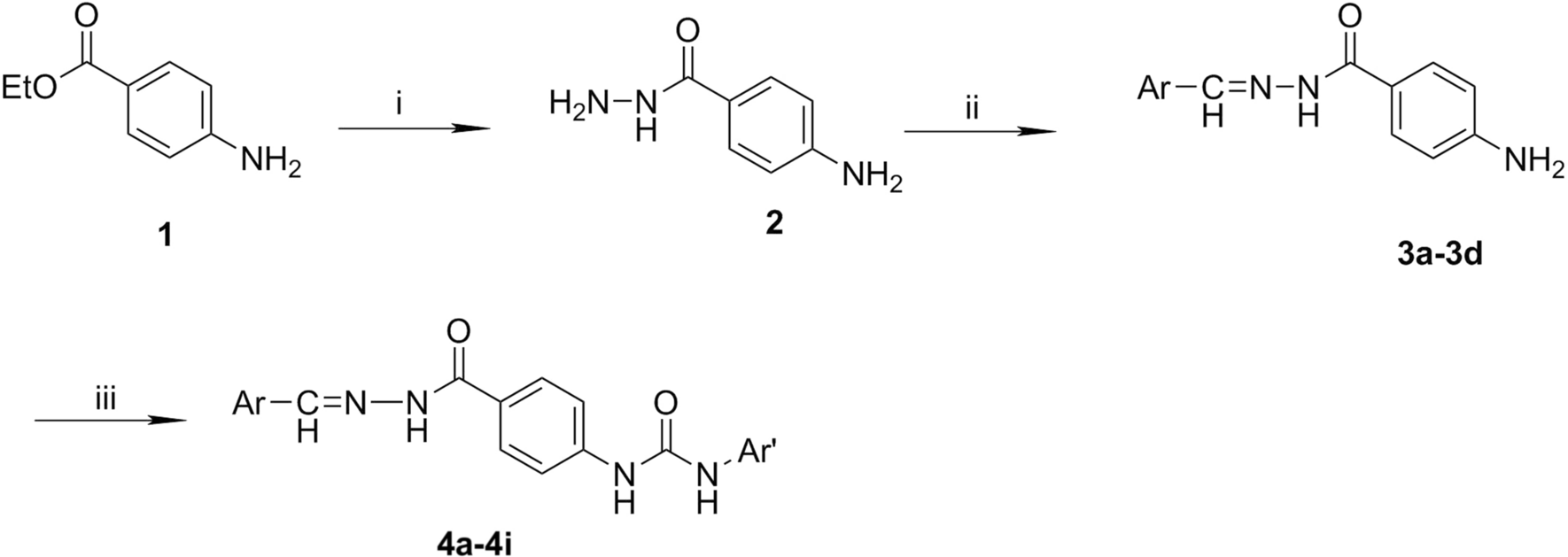
Access to Dihydroquinazolinones, spiro‐Quinazolinones and their Bioactive Molecular Scaffolds by Exploring the Unique Reactivity of 2‐Nitrobenzonitrile towards Cu‐Hydrazine Hydrate - Sahoo - 2023 - ChemistrySelect - Wiley Online Library

eHydrogenation: Hydrogen‐free Electrochemical Hydrogenation - Russo - 2023 - Angewandte Chemie International Edition - Wiley Online Library

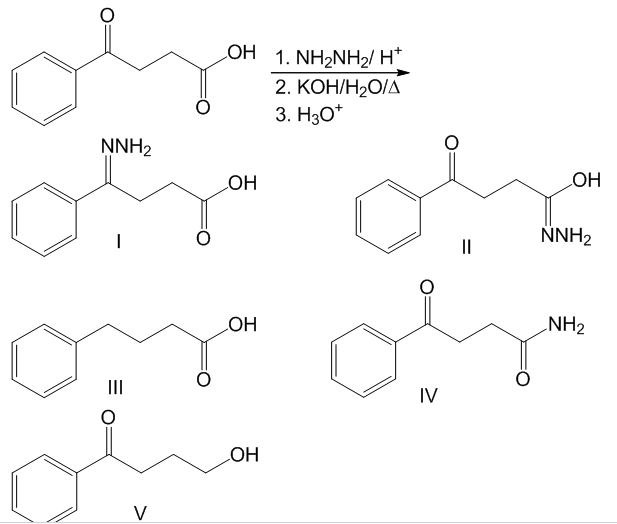
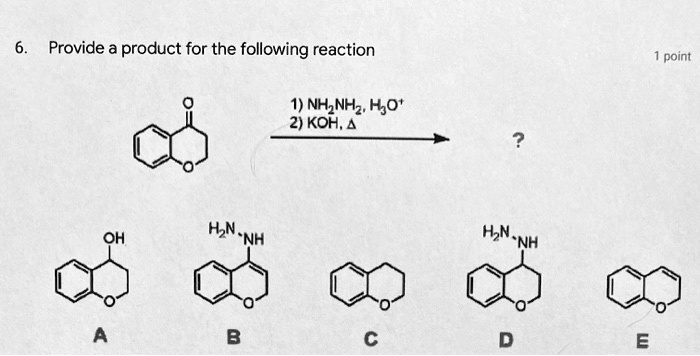
SOLVED: Reagents, conditions, and products as appropriate: A 1 NH2NH2 cat. HCl, -H2O CH3CH2NH2 cat. HCl, -H2O 2) KOH, heat 2 (2 pts) Imines hydrolyze in acidic aqueous media to form ketones

Figure 3. Synthesis routes of sulfone derivatives containing phenoxymethyl and 1,3,4-oxadiazole moiety. Reaction conditions and reagents: (a) MeOH, 98%H2SO4, reflux 5h; (b) NH2NH2▫H2O, EtOH, 25°C–reflux, 1h; (c)KOH, CS2, EtOH, 25-46-76°C, 7h; (d)

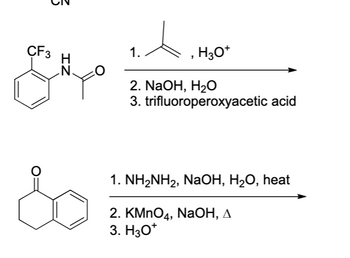
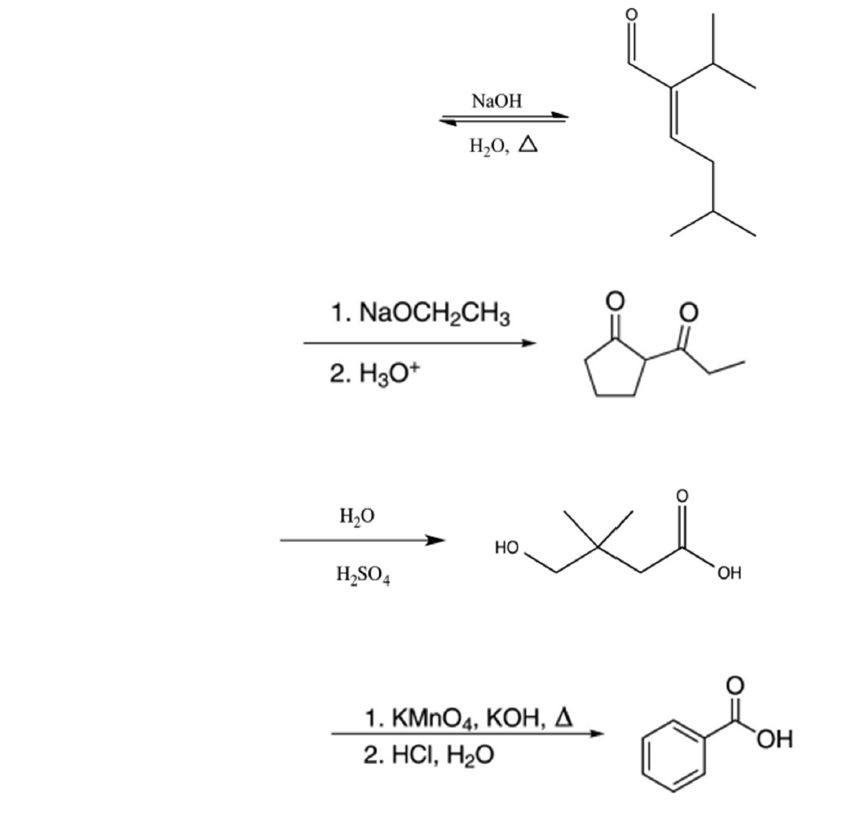
OneClass: Propose a detailed arrow pushing mechanism for (e), (i), and (s). 1. catalytic Ho NH2NH2 (p...

Synthesis of the target compounds 24-43. Reagents and conditions: (a)... | Download Scientific Diagram
HYDRAZINE HYDRATE Extra Pure | Laboratory chemical suppliers, Laboratory Chemicals, Lab chemical distributors, Laboratory chemicals manufacturer, Lab chemical supplier, Lab chemicals exporter, Lab chemical manufacturer, Alpha Chemika India.
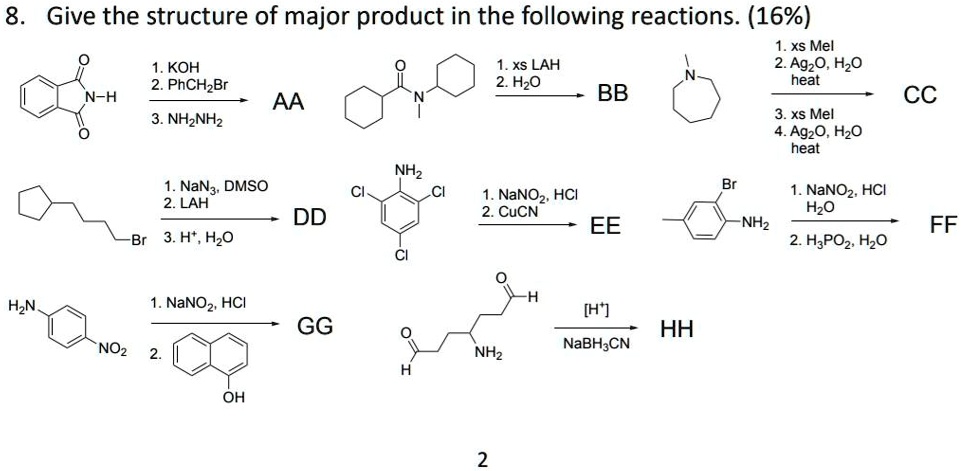
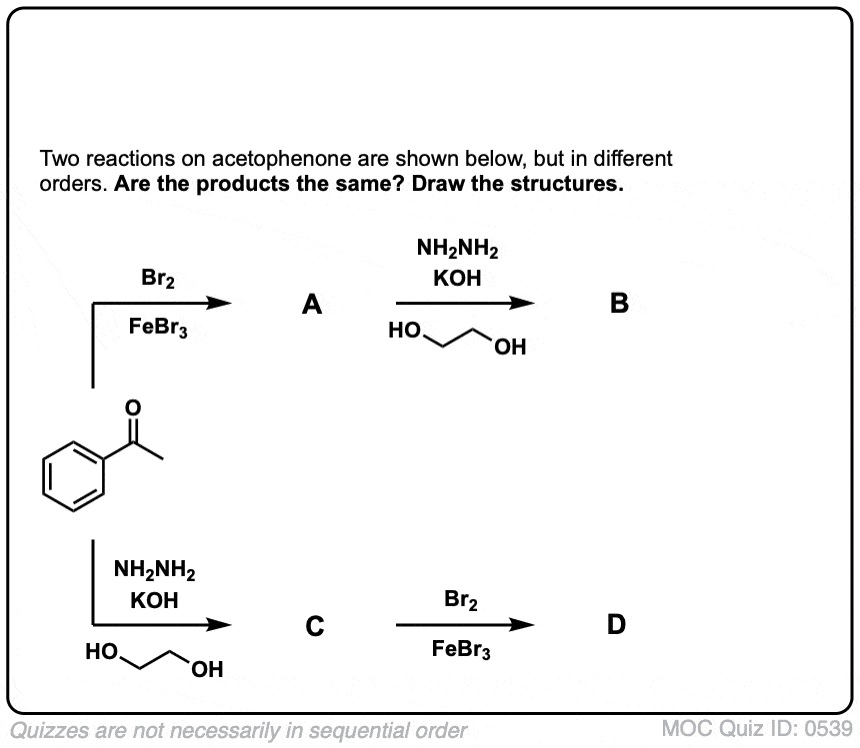
SOLVED: Give the structure of the major product in the following reactions. (16% 1.xs MeI 1.KOH 1.xs LAH 2.AgO, H2O 2.PhCHBr 2.H2O heat BB AA CC 3. NH2NH2 3.xs MeI 4.Ag2O, H2O heat

Reagents and conditions: (a) EtOH, NaHCO3, reflux, 24h; (b) NH2NH2·H2O,... | Download Scientific Diagram
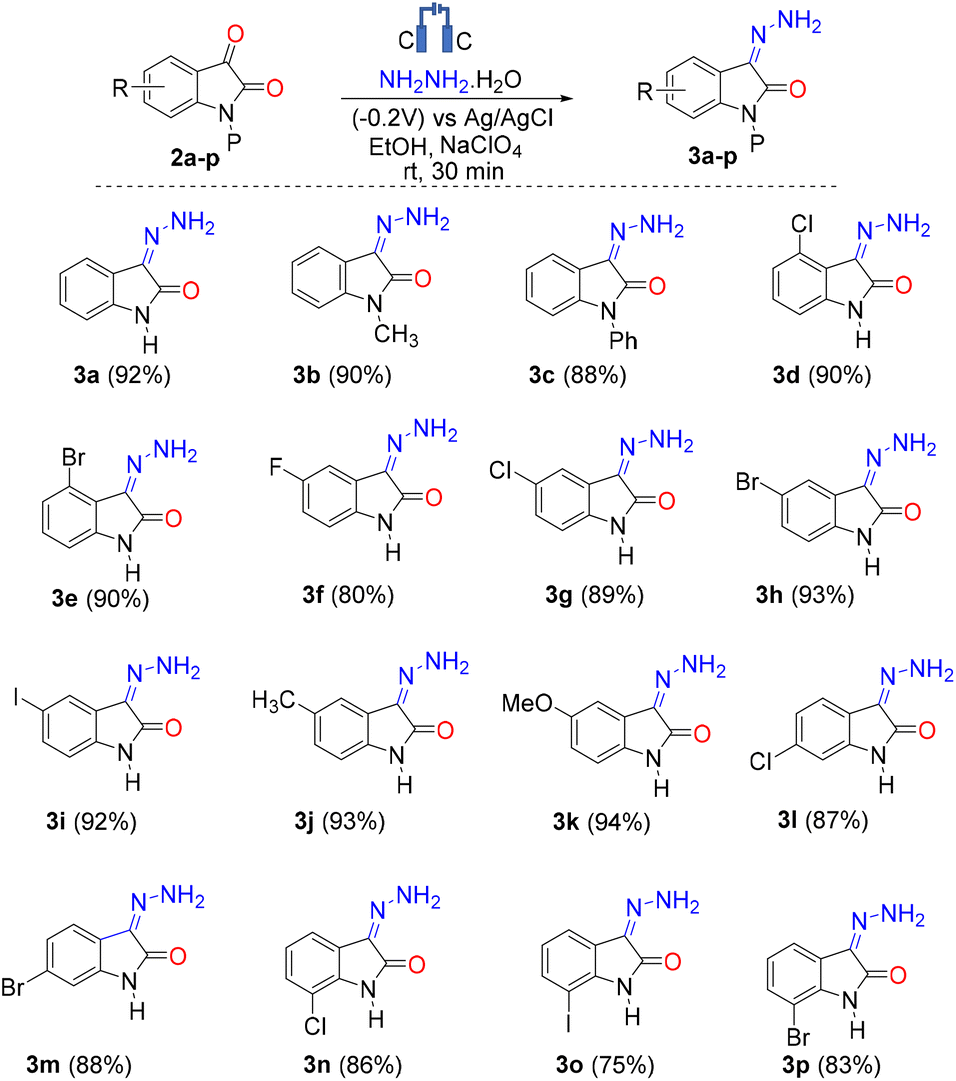
Electro-organic synthesis of isatins and hydrazones through C–N cross-coupling and C(sp 2 )–H/C(sp 3 )–H functionalization - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D3OB01128C

Development and Scale-Up of a Continuous Manufacturing Process for a Hydrazine Condensation Reaction | Organic Process Research & Development

Figure 2. Synthesis routes of sulfone derivatives containing 1,3,4-oxadiazole moiety. Reaction conditions and reagents: (a) MeOH, 98%H2SO4, reflux 5h; (b) NH2NH2▫H2O, EtOH, 25°C–reflux, 4h; (c) KOH, CS2, EtOH, 25-46-76°C, 7h; (d) NaOH,

Functionalized 3-hydroxy-3-aminoquinoline-oxindole hybrids as promising dual-function anti-plasmodials - ScienceDirect